Randomised controlled trial (feasibility study) of prophylactic pyloric balloon dilation during Ivor Lewis oesophagectomy to prevent delayed gastric emptying
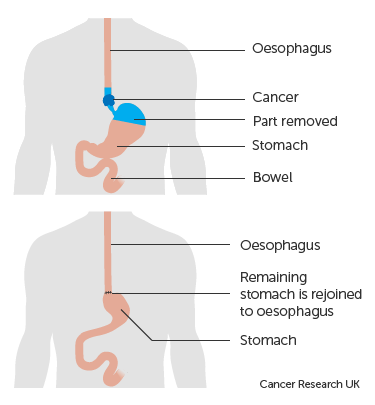
The Ivor Lewis oesophagectomy is a surgical procedure where cancer within the oesophagus and stomach is removed before the remaining  stomach is brought into the chest and joined to the remaining oesophagus. In around 37% of people, this procedure can result in a complication called delayed gastric emptying (DGE) which causes vomiting, difficulty swallowing, regurgitation, malnutrition, anastomic leak, aspiration pneumonia and a longer hospital stay.
stomach is brought into the chest and joined to the remaining oesophagus. In around 37% of people, this procedure can result in a complication called delayed gastric emptying (DGE) which causes vomiting, difficulty swallowing, regurgitation, malnutrition, anastomic leak, aspiration pneumonia and a longer hospital stay.
It is not fully understood what causes DGE and while there are treatments, none are preventative. Mr David Chan, Consultant Upper GI Surgeon has developed a study in collaboration with Mr Mohamed Abdelrahman, Research Fellow to determine whether DGE can be prevented by  inserting a 20mm balloon into the opening of the stomach via endoscope, at the time of an Ivor Lewis oesophagectomy.
inserting a 20mm balloon into the opening of the stomach via endoscope, at the time of an Ivor Lewis oesophagectomy.
Over the course of 12 months, 24 – 32 participants will be recruited and randomly allocated to one of two groups. One group will receive normal clinical care while the other group will receive normal clinical care with the addition of a 20mm balloon. All participants will be followed up regularly and asked to complete questionnaires relating to DGE symptoms. The results from this feasibility study will be used to provide evidence on whether a larger version of the study with several sites can be conducted, and test whether the intervention is successful and can be used as a preventative treatment in the future.



